Resource Management
6. Resource Management.................................. 142
6.1 Provision of Resources................................ 143
6.2 Human Resources........................................ 146
6.3 Infrastructure................................................ 155
6.4 Work Environment and Contamination Control (combining 6.4.1 and 6.4.2)................. 158
Logically, after management sets the expectations and direction along with their goals, you must provide resources to achieve what your organization committed to. Section 6 covers a variety of planning issues including the people needed, the infrastructure and the work environment, and depending on your commodity and product you can easily be influenced by all of these factors.
While we may speak in other sections about “How to achieve it” and “How to prove it” many of these things will only be achieved and proved through visually going and “seeing.” As an example:
- You can only really check the adequacy of the work area by observing the work area
- Checking that the right people have been provided requires you to look at numerous factors to diagnose whether or not the company has done their due diligence and provided the people
So, in short, what we are saying in this section is do not look for any real new or innovative techniques to achieve these basic things, such as the resources to your planning. Focus on the fundamentals, such as 5S, cleanliness, filling open positions left vacant by attrition, training to develop new skills for the new jobs you undertook, etc.
Having the right resources, especially human resources, to ensure that you can implement and maintain your quality system is the most critical element for your company’s success. The right human resources will ensure the system is sustainable and will be able to quickly identify areas of concern.
Employees who are not appropriately qualified and trained for the roles they assume can result in untold damage. As an example, a well-known FDA-regulated organization received warning letters, was then placed under a consent decree by a US Federal Court, and ultimately had to close its doors due to the financial strains of their quality system issues. Their issues all started because the person responsible for facility and equipment maintenance did not fully understand the critical need to follow the required maintenance schedules strictly as written, resulting in equipment and systems falling out of a validated state. All product manufactured under those non-validated processes had to be recalled and destroyed, and the company was unable to recover from such an overwhelming loss.
6.1 Provision of Resources
 What is it?
What is it?
This section refers to the need for organizations to back their plans with the resources required to execute and maintain the QMS. While we think of resource traditionally in the monetary sense, it is more about people, infrastructure, and the more tangible items. After all, money is merely the exchange medium for goods and services. No business has ever made parts out of money; however they have made goods from the machines purchased with money. In this section:
- You have to provide the resources to maintain the effectiveness of the QMS
- Provide adequate resources to meet regulatory requirements
These resources are used to bring the level of the organization up to the level of EFFECTIVENESS, and so it is essential that the level of resources provided meets the performance expected. Resources allocated at less than the necessary level will certainly show in a reduced performance level. The standard is not asking you to OVER-COMMIT resource, but to RIGHT SIZE the resource to meet your obligations.
Objective
- Get the resources in place to the level of effectiveness expected by the organization’s customers and regulators
How do I do it?
- Refer to the goals and objectives set out for the organization in Section 5
- Determine the reason you may not be meeting these goals; Is it staff? Is it technical? Is it knowledge? Is it equipment? Where is the gap?
- Develop a resource plan to address the gap, do not over commit resources, but right size
- Bring the resource plan to the management table along with clear paths to close the gap, remember the resource must be properly funded in order to deliver closure of the gap. Document the decisions and the effect the resource has on the goals as they are met
Tools and Techniques to achieve it
- Strategic and operational planning
- Balanced scorecard methodology
Documents you can use to prove it
- Management Review meeting minutes
- Dashboards showing performance to expected levels
- Operational plans backed by actions showing resource consideration
- Skills/technology gap assessments for new programs considered and the actions to fill those gaps
Questions to ensure you know it
- When we bring on a new piece of work, how do we determine what new skills and resources we need from the people perspective?
- For objectives not being met currently, are any of the issues due to failure to provide the right resources, and if so can I put my finger on several instances of the same gap of the same resource elsewhere?
- How do we bring up requests for resources to those that have responsibility to provide them?
How can you fail at it?
- You can fail to provide the resource
- You can fail to link the resource to the performance gap that it addresses
- You can fail to update the resource needs as new programs are added
6.2 Human Resources
 What is it?
What is it?
This section deals with the training, competency, and awareness of the individuals doing work that affects product quality. Notice that it does not say floor level workers, it is not only hourly, or manufacturing workers but your buyer could also affect product quality.
You have to have a documented process for establishing competence, providing training, and assuring awareness. You have to:
- Establish competence criteria
- Provide training to get the competence
- Evaluate the competence
- Ensure the awareness
- Document the proof
What is competence? (see ISO definition, summarized here). It is the APPLICATION of knowledge to achieve the result. So training IS NOT competence. You can be trained and still be incompetent. Competence MUST be backed up by the ability to achieve intended results.
As an example:
If an associate is responsible to run a process at 5% scrap and a 30 second cycle time, and they run 8% scrap and 40 seconds cycle time...they are trained but not at all competent.
In Sinek’s book he explored why some companies are more successful than others, and the summary is the premise that:
- Everyone knows WHAT they do
- Some people understand HOW they do it
- Few people understand WHY they do what they do

And so Sinek hits on the fact that the most successful companies connect WHY they want their employees to do what they do. This can become equivalent to the relationship between training, competency, and awareness:
- Training describes WHAT you want them to do
- Competency describes HOW you want them to do it (defined application of training)
- Awareness connects with WHY you want them to do it
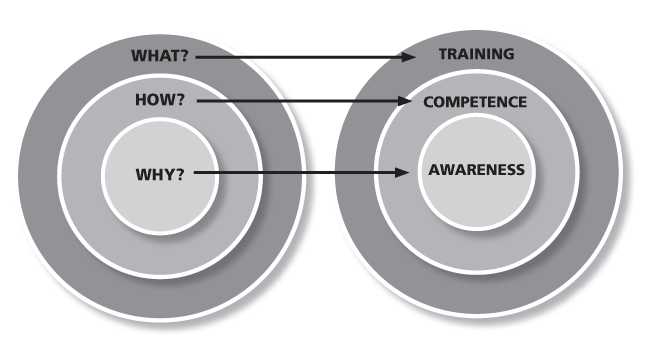
In short, communicating awareness is the bridge between mere compliance to a request, and actual engagement of an employee. The employee has to internalize your cause and be able to understand the implications of WHY.
Notice that the standard requires the level of competency and training to be tied to the risk; there is no flat rate standard when it comes to competency.
Objective
- Make certain people can do what you ask them to do, to the level you ask them to do it, and know WHY they are doing it.
How do I do it?
- Define what you need the people to be able to competently do once they have finished training…it must be measurable
- Provide the training
- Evaluate the training
- Tell them WHY it matters that they do what they do correctly...what is the effect
- Retain the documentation of the above activities
Tools and Techniques to achieve it
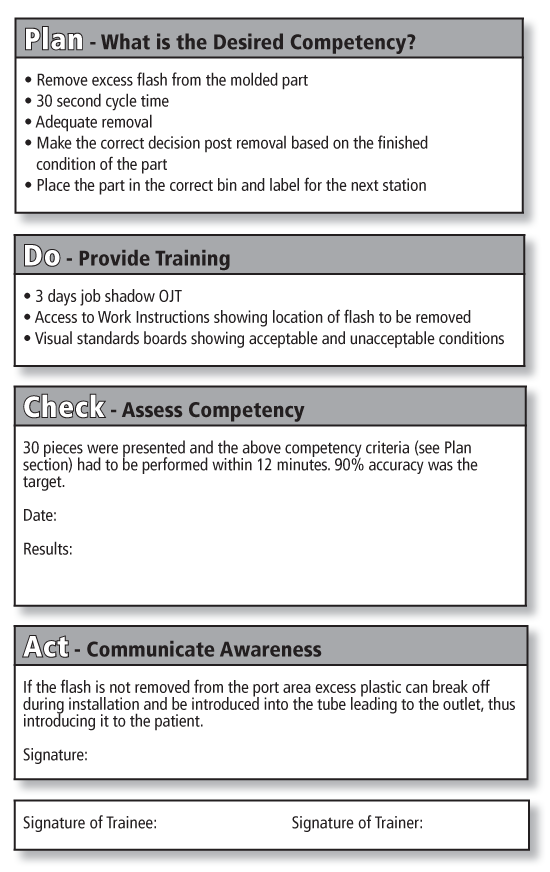
- PDCA methodology as it applies to competency. This is a new use of a long standing methodology where we apply the PDCA cycle to competency evaluation (see figure 6.3)
- P - Plan - What competency do they need?
- D - Do - What training do you give them?
- C - Check - Check the training to make sure they can perform
- A - Act - What happens if they do not do it correctly? (Awareness)
Notice how proper use of the PDCA in the example in figure 6.3 assures that you have the documentation you need to assure competency is properly done.
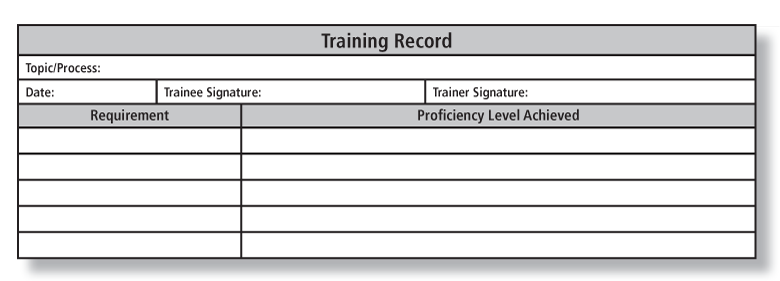
Documents you can use to prove it
- Training records when clear competence is displayed
- Performance reviews that tie back to competence performance
- Line meeting minutes showing awareness communication
- The process created to meet the requirement
Questions to ensure you know it
- When I hire a new person, how do I assure they are not just trained, but competent?
- Considering the performance of my existing staff, is this the level of competence I want from them, and if so how would I numerically express it?
- How do I make the people working with me aware of WHY what they do is important?
- If I went out on 2nd shift to line 5...how would I know the operator working there is competent, and if I know it because I know it...can I prove it?
How can you fail at it?
- You can train the people and not validate that the training was effective
- You can train the people and not tell them WHY it matters
- You can only train the floor associates and forget about the support services in your facility
- The documentation you provide does not show HOW you judged them to be competent
6.3 Infrastructure
 What is it?
What is it?
Unlike other standards, in ISO 13485 you have to document the level of infrastructure required for what it is you do and the corresponding requirements to get you to where you want to go. Many times this is shown through contracts, as you usually have to quote these infrastructure items in order to make the product. These things can include:
- Workspaces and utility requirements as well as processing equipment and transportation processes
- The maintenance activities, including time interval if the maintenance may endanger the product. This idea of “maintenance” can apply to production equipment, work environment, and even extend to monitoring and measuring equipment
- You have to maintain records of this maintenance
Objective
- Set a level of requirement for the supporting items essential to making the product or service, and then provide maintenance to make certain that the level does not slip
How do I do it?
- Determine the things that will impact your ability to produce the part (see tools below)
- Determine the requirements for those things, such as humidity levels, cleanliness levels, etc.
- Document these things in a place where you will use them again, such as in the contract
- Develop controls to monitor if these things slip or deviate
- Develop maintenance of these things, and monitor the maintenance appropriately
- Retain the records of the maintenance
Tools and Techniques to achieve it
- Risk Management to evaluate
- Turtle methodology (see the WHAT box, these are infrastructure items)
- Control plan methodology for environmental controls of process
- Predictive maintenance processes and programs
- Software systems to manage maintenance
Documents you can use to prove it
- Contracts showing required levels of infrastructure items
- Completed control plans showing monitoring of these controls
- Maintenance logs and schedules
Questions to ensure you know it
- How do I know what is an acceptable work environment for lighting given my product, and if there is a standard...how would I find out?
- What are my maintenance technicians checking on the facilities, machines, and monitoring devices?
- Is the maintenance that is being performed showing positive results in the product or can I find multiple instances of maintenance related nonconformities?
- How many times has the facility caused issues in my product?
How can you fail at it?
- You can fail to schedule maintenance of the infrastructure at an interval that prevents issues
- You can fail to identify the right level of infrastructure for the expected level of performance (i.e. clean rooms not rated for the appropriate level of cleanliness)
- You can only perform maintenance on the process equipment and forget the supporting items like forklifts, hoists, and work-in-process areas
6.4 Work Environment and Contamination Control (combining 6.4.1 and 6.4.2)
 What is it?
What is it?
In this area the standard speaks about the requirements for the work area needing to meet product requirements, and in 6.4.2 quite specifically contamination control. We combined them here because contamination control is simply the prescription of the work environment. In this section the organization has to:
- Document the requirements for the work environment and the PROCEDURES to monitor it (notice one is about achieving the requirement, the other is about maintaining the set requirement)
- Document the requirements for health, cleanliness, and clothing, if it is applicable based on the commodity, and make these requirements applicable to full time and temporary workers who are competent or supervised by a competent person
- If products are suspected or confirmed to be contaminated you have to document the procedures to make sure the product and contamination does not creep into the work environment or other products
- If you are manufacturing sterile devices you have to document control of contamination from microorganisms and maintain the required cleanliness throughout all processes
Objective
- Keep the work environment clean to the appropriate level and IF the level is breached, know what to do to quarantine and isolate the threat
How do I do it?
- Determine the requirements for the work areas, using risk management techniques
- Determine the monitoring controls and place in the control plan
- Determine the health, cleanliness, and clothing requirements IF these things impact your commodity
- Adjust the training plan to cover both full time competent people and temporary contract workers, and implement a mentoring, job shadowing policy, for anyone NOT meeting appropriate proficiency levels for the tasks/areas
- Implement and document a procedure for the identification, quarantine, and recovery of contaminated material that MAY come in contact with the line
Tools and Techniques to achieve it
- Risk Management to evaluate needs
- Regulatory requirements that may be specific to your device (requirements established in your premarket clearance, industry standards, etc.)
- Nonconforming material process/procedure (which may include a Nonconforming Material Review Board to provide oversight for more critical products/processes)
- Control plan methodology
- 5S programs
- Reaction plans
Documents you can use to prove it
- Requirements for cleanliness communicated in operator instructions and throughout the facility
- Visual boards showing acceptable standards and audits of these standards
- Control plans for the environment and the corresponding records
- Nonconforming material records, investigations, and final product dispositions
- Internal audit reports assessing the work environment against regulatory requirements and company procedures
Questions to ensure you know it
- Can I go out onto the floor right now and find the newest person in my controlled area, and if they are not competent, can I link their role to the mentor they have been assigned?
- For the workspaces out on the shop floor, if I was to approach them, do I know what clothing and standards of cleanliness are acceptable prior to beginning work?
- If a product becomes contaminated, how do I segregate it and bring the resulting workspace back up to the standard level; keeping the contamination from other product at the station?
How can you fail at it?
- You can fail to control the temporary people working in areas requiring special precautions
- You can fail to have a contamination plan for a product if required
- You can fail to have the right level control on the workspaces
Proper and adequate resource management is the foundation of your quality system and critical to your ability to produce safe, effective, and compliant medical devices. Consideration of each element of this requirement should be viewed through the lens of risk management techniques to ensure proper weight is given as applicable. All such evaluations should be documented, and sound rationales developed for any decisions that stray outside of any industry standards or regulatory requirements. The extra time and effort you put into this section of your quality system will be returned two-fold by ensuring a sound foundation upon which you will build.